In-Depth Sharing | How to Produce High-Quality IVD Core Enzyme Raw Materials—Antibody Modified Hot Start Taq DNA Polymerase
COVID-19 swept the globe in recent two years, and molecular diagnostics stood at the center of the world stage. As a gold standard for SARS-Cov-2 nucleic acid detection, fluorescence PCR method provides a powerful guarantee for SARS-Cov-2 detection. However, the problems of inaccurate test results, false positive and false negative also once made the molecular diagnosis technology controversial. In addition to human operation factors, the quality of the kit should be considered in the final analysis. The core component of the SARS-Cov-2 detection process—the hot start enzyme in the amplification module is undoubtedly the most critical link.
The enzymes in the amplification module of diagnostic kit on the market are basically antibody modified hot start polymerase, but the enzyme raw materials of different manufacturers vary greatly in the actual sample detection. So why choose antibody modified hot start Taq polymerase as the core enzyme of IVD, how to evaluate its quality, and how to screen high-quality antibody modified hot start Taq polymerase?
Why Choose Antibody Modified Taq DNA Polymerase as the Core Enzyme of IVD?
Hot start Taq DNA polymerase is widely used in the field of IVD, which solves the problems of nonspecific amplification caused by primer dimer or mismatch in conventional PCR technology, greatly improves the specificity of the reaction, and realizes accurate detection. At present, the common hot start Taq DNA polymerase in the market are mainly divided into chemical modification, ligand modification and antibody modification. These three methods are different in principle and have their own advantages and disadvantages (see Table 1).
|
Enzyme Activity Blocking Type |
Chemical Modification |
Ligand Modification |
Antibody Modification |
|---|---|---|---|
|
Principle |
The small chemical molecules allow the formation of cross-links inside the DNA polymerase protein to block the polymerase activity. |
Nucleotide aptamer binds to polymerase through non-covalent bond to inhibit polymerase activity. |
Anti-Taq antibodies bind to Taq polymerase and inhibit polymerase activity. |
|
Enzyme Activity Release Conditions |
Temperature:95 ℃ Time:5-10 min |
Temperature:95 ℃ Time:30 s-1 min |
Temperature:95 ℃ Time:30 s-1 min |
|
Advantages |
The enzyme activity remains more stable without any foreign DNA pollution, and the cost performance is higher. |
No activation step is required, and the stability is high, reducing the possibility of sample degradation. |
The enzyme activation time is short, ensuring enzyme activity, strong affinity, and high sensitivity. |
|
Disadvantages |
Enzyme activation for a long time may affect the activity of the enzyme, resulting in a decrease in the yield of PCR products. |
The nucleic acid ligand is a reversible inhibitor, which is not as stable as the chemical modification method and is not specific enough. |
Antibody modification is a dynamic balance, antibody production costs are high, and the reagent mix optimization time is long. |
Table 1. Comparison of Advantages and Disadvantages of Common Modification Methods of Hot Start Taq DNA Polymerase
In contrast, chemical modifiers can not be synthesized in batch, and it takes a long activation time for DNA polymerase to release enzyme activity; The effect of ligand modified DNA polymerase is good only at 20-25°C, and the activity of polymerase is released at 40°C, that is, the blocking effect of enzyme activity is unstable and the specificity is not good; The antibody modified hot start polymerase can achieve a good effect of blocking enzyme activity, that is, the corresponding blocking effect is the best, and the release speed of enzyme activity is fast, which can greatly reduce the reaction time of PCR. Therefore, it is the most widely used DNA polymerase hot start transformation method in the IVD market.
Evaluation Index of Antibody Modified Hot Start Taq DNA Polymerase
Enzyme activity, amplification efficiency, sensitivity and antibody blocking effect of Taq DNA Polymerase are the main evaluation indicators for antibody modification hot-start enzymes.
Enzyme Activity of Taq Polymerase
There is currently no fixed standard for the enzyme activity determination of Taq polymerase in the molecular diagnostic industry, and different manufacturers have different enzyme activity calibration methods, which may cause major problems in the use of customers. The general test method is the isotope method for determination. This method has high sensitivity, but can only detect polymerase activity, and sometimes does not reflect the actual amplification effect of TaqMan probes. TaqMan probe method qPCR requires not only the 5'→3' polymerase activity of the Taq DNA polymerase, but also the 5'→3' exonuclease activity. This is the defect of conventional Taq polymerase activity determination. It is recommended that after purchasing the Taq polymerase of a specific company, you need to use your own designed primer probe to perform a second calibration of the actual enzyme activity to reduce the uncertainty in the application. In addition to the 5'→3' polymerase activity detection of the Taq DNA polymerase, Yeasen Biotechnology (Shanghai) Co., Ltd. (hereafter called Yeasen) also uses the TaqMan method to perform the 5'→3' exonuclease activity calibration, which reduces the problems in the actual application of customers.
Amplification Efficiency
The amplification efficiency of PCR is closely related to the performance of DNA polymerase. Generally, the amplification efficiency is verified by diluting the template into a series of concentration gradients for qPCR reaction, and making a standard curve for the Ct value of each diluted sample with the log value of the initial amount of the template (or the dilution multiple of the unknown amount sample), so as to obtain the slope and R2 value. The amplification efficiency is calculated by the formula e = 10-1 / slope-1. If it is between 90 ~ 110%, it means that the amplification is normal. If < 90%, the whole reaction conditions of qPCR may be inappropriate or the primer design may be improper.
The amplification efficiency is closely related to the specific activity of the polymerase. The higher the purity of the polymerase, the higher the specific activity, which can reduce the combination of inactive Taq polymerase and substrate and improve the probability of effective amplification.
Sensitivity
The sensitivity of Taq polymerase is closely related to many aspects. In addition to the enzyme purity mentioned above, another aspect is the affinity between Taq polymerase and substrate. If the affinity is higher, the sensitivity will naturally be higher. Compared with the Taq polymerase with low substrate affinity, it is necessary to improve the sensitivity by increasing the enzyme concentration, that is, increasing the molecular number of Taq polymerase in a single reaction. At this time, the reagent cost will increase a lot. Yeasen ensured the sensitivity of single bit copy number detection by screening high affinity Taq mutants.
Antibody Blocking Effect
Hot start Taq polymerase is one of the most important factors to improve the sensitivity and specificity of PCR amplification. Non hot start Taq polymerase will have certain activity in the process of reagent preparation, which is easy to lead to non-specific amplification and eventually reduce the sensitivity. An active and effectively blocked Taq polymerase is very important for the effective amplification of qPCR. Through high-throughput screening of antibodies, Yeasen obtained antibodies with high affinity and easy release, and developed relevant detection methods to ensure the high quality of hot start Taq polymerase.
1. Melting Curve Determination
The non-hot-start group, antibody blocking group and polymerase free control group were set.The templates and primer pairs were added to the reaction system of SYBR green dye qPCR, incubated at 37 ℃ for 40 min, and the melting curves were detected respectively.
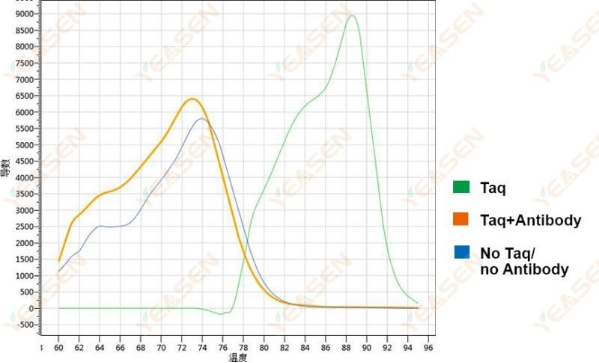
Figure 1. Melting curve of Taq antibody sealing effect
The non-hot-start group showed the production of amplification products, while the other two groups were primer dimers without product formation. This shows that the antibody blocking effect is obvious.
2. Fluorescence Quantitative Method
Generally, the blocking effect is judged by comparing the fluorescence values of non hot start group, antibody blocking group and non polymerase control group. The extension was carried out at 50℃. After the reaction, the product was taken, stained with an appropriate amount of PicoGreen staining solution, and then the fluorescence signal was detected. According to the fluorescence value to judge the blocking effect of hot start polymerase activity and further calculate the blocking efficiency, it is generally considered that the blocking effect is good if the blocking efficiency is greater than 90%.
|
Group |
Positive Control |
Negative Control |
Antibody Modified Hot Start Polymerase 1 |
Antibody Modified Hot Start Polymerase 2 |
|---|---|---|---|---|
|
Fluorescence Value |
56750 |
3250 |
5000 |
5450 |
|
Blocking Efficiency |
/ |
/ |
96.73% |
95.89% |
Table 2. Test of Blocking Efficiency of Taq Antibody
Note: Blocking efficiency = 100% - (sample fluorescence value - negative fluorescence value) / (positive fluorescence value - negative fluorescence value).
How to Produce High-Quality Antibody Modified Hot Start Taq Polymerase?
The production of qualified IVD reagents is generally strictly controlled in several links such as raw material control, production management, quality inspection control, warehousing and transportation. As the core raw material of IVD reagent, Antibody modified hot start Taq polymerase mainly considers two core components: blocking antibody and DNA polymerase. Therefore, we also need to be cautious in raw material control, production management, quality inspection control, warehousing and transportation.
Raw Material Control
Strict raw material quality management standards, including procurement standards and preservation standards. Each batch of important raw materials is quality inspected and samples are kept for storage. The core material of antibody modified hot start Taq polymerase: blocking antibody and supporting buffer ions. In terms of raw material control, the main attention is to avoid the introduction of exogenous contamination, and the screening of blocking antibodies requires further detection of host DNA residues in addition to ensuring the purity of the antibodies.
Production Management
The production process is managed in strict accordance with IVD product production standards. From sample research and development to large-scale production, the production process is stabilized and batch stability is ensured. At the same time, strictly control the packaging process. The production of antibody modified hot start Taq polymerase needs to be accurately proportioned to ensure product quality according to the purity of the antibody and the enzyme activity of the DNA polymerase.
Quality Control
Product quality control shall strictly follow the "triple protection": quality inspection of intermediate raw materials, self inspection and sample retention of finished products, and sampling control of finished products. The quality control link after the production of antibody hot start enzyme mainly focuses on the detection of nuclease residue, E. coli, DNA amplification ability and the sealing effect of antibody modified hot start Taq polymerase. At the same time, it adheres to the "zero tolerance" of quality problems in the stability control of different batches.
Warehousing and Transportation
Follow the principles of modular warehousing and cold chain transportation.
Yeasen Provides High Quality IVD Core Enzyme Raw Materials
As a national high-tech enterprise focusing on the R&D and production of molecular enzyme raw materials and Library Building Kits for a long time, the enzyme raw materials of Yeasen can be used in the field of IVD molecular diagnosis and treatment by relying on the long-term reserve of molecular enzyme two-way transformation technology, protein fermentation and purification technology and high-efficiency antibody screening technology platform.
Yeasen can not only provide blocking Taq antibody and Taq 5'→ 3' exonuclease monoclonal antibody, but also provide core reagent for RT-PCR detection kit assembly and a series of key molecular enzyme raw materials required, including antibody modified hot start Taq polymerase, reverse transcriptase, RNase inhibitor and UDG/dUTP.