Hieff NGS™ Ultima Dual-mode RNA Library Prep Kit
Product Description
Hieff NGS™ Ultima Dual-mode RNA Library Prep Kit is a total RNA sequencing library construction kit for the Illumina® and MGI® sequencing platform, including RNA fragmentation reagents, reverse transcription reagents, conventional and strand-specific ds-cDNA synthesis reagents, and library amplification reagents. The sequencing library can be constructed followed by the mRNA purification kit or rRNA removal kit. The two-strand synthesis module is equipped with two buffers to meet the need for a conventional library or strand-specific library. Among them, dTTP is replaced with dUTP in the strand-specific two-strand synthesis Buffer, so dUTP can be added to the second strand of cDNA. The high-fidelity DNA polymerase used in this kit cannot amplify the DNA template containing uracil, achieving strand specificity. All reagents provided have undergone strict quality control and functional verification, ensuring the stability and reproducibility of library construction to the greatest extent.
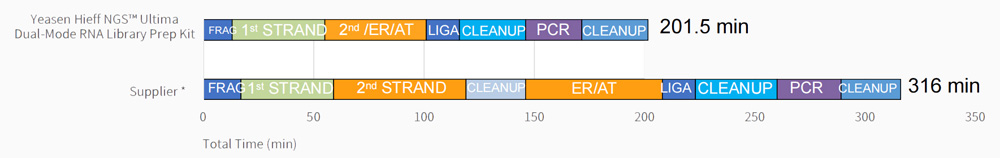
Work flow
Product Components
|
Components |
12308ES24 |
12308ES96 |
|||
|
12308-A |
2× Frag/Prime Buffer |
250 μL |
930 μL |
||
|
12308-B |
1st Strand Enzyme Mix |
48 μL |
192 μL |
||
|
12308-C |
Strand Specificity Reagent |
150 μL |
580 μL |
||
|
12308-D |
2nd Strand Buffer (dNTP) |
720 μL |
2×1440 μL |
||
|
12308-E |
2nd Strand Buffer (dUTP) |
720 μL |
2×1440 μL |
||
|
12308-F |
2nd Strand Enzyme Master Mix |
120 μL |
480 μL |
||
|
12308-G |
Ligation Enhancer |
720 μL |
2×1440 μL |
||
|
12308-H |
Novel T4 DNA Ligase |
120 μL |
480 μL |
||
|
12308-I |
2×Super Canace® II High-Fidelity Mix |
600 μL |
2×1200 μL |
||
|
12308-K |
Nuclease Free H2O |
300 μL |
1000 μL |
||
Note: This kit is compatible with both Illumina and MGI platforms, but additional Illumina or MGI Primer Mix ( Cat# 13335 Primer Mix for Illumina and Cat# 13334 Primer Mix for MGI ) is required.
Shipping and Storage
The Hieff NGS™ Ultima Dual-mode mRNA Library Prep Kit components in Box I are shipped with ice packs and can be stored at 2-8°C for one year.
The Hieff NGS™ Ultima Dual-mode mRNA Library Prep Kit components in Box II are shipped with dry ice and can be stored at -20°C for one year.
Cautions
1 Operation
1.1 For your safety and health, please wear personal protective equipment (PPE), such as laboratory coats and disposable gloves, when operating with this product. This product is for research use ONLY!
1.2 Thaw components at room temperature. Mix thoroughly by inverting up and down several times, spin down briefly and place on ice for use.
1.3 It is recommended to perform each step reaction in a thermal cycler with a heated lid. The thermal cycler should be preheated to the set temperature prior to use.
1.4 Supplies free of RNase contamination and cleaning the experimental area regularly are necessary.
1.5 Improper operations may very likely cause aerosol contaminations, impacting the accuracy of the result. Mandatory physical isolation of PCR reaction mixing regions and PCR product purification assay regions is recommended. Equipped with equipment such as specialized pipettes for library construction.
2 Adapter Ligation
2.1 Illumina or MGI Long Adapter (Barcoded Adapter) kits and short Adapter kits are available for customers to choose from according to their experimental requirements.
2.2 Selecting high-quality, commercial adapters was recommended. If self-made adapters are selected, please entrust a company with experience in NGS primer synthesis and remark on the need for strict contamination control. In addition, it is recommended to prepare DNA annealing solution in a clean bench and only operate one type of adapter each time to prevent cross-contamination.
2.3 Please thaw the adapters on the ice or at 4°C; when operating at room temperature, the laboratory temperature should not exceed 25°C to prevent the adapters from denaturing.
2.4 The concentration of the adapter directly affects the ligation efficiency and library yield. The adapter volume added to the kit is fixed to 5 μl. The adapters are recommended to be diluted with 0.1×TE buffer and the diluted adapters can be stored at 4°C for 48 hours. Table 1 lists the recommended adapter amount for different amounts of input RNA.
Table 1-1 The recommended Illumina adapter amount for different input RNA
|
Input Total RNA |
Illumina Adapter stock concentration |
|
10 ng |
1 μM |
|
100 ng |
1.5 μM |
|
500 ng |
3 μM |
|
≥1 μg |
5 μM |
Table 1-2 The recommended MGI® adapter amount for different input RNA
|
Input Total RNA |
MGI Adapter stock concentration |
|
100-499 ng |
2 μM |
|
500-4000 ng |
5 μM |
*The Adapter usage can be adjusted according to different types of Total RNA samples and input amount.
3 Library Amplification
3.1 On the basis of the first-generation DNA polymerase, the high-fidelity DNA polymerase in the kit has greatly improved its amplification uniformity and exhibits no amplification bias.
3.2 If Indexed Adapter (also known as a long adapter or large Y adapter) is ligated to the target DNA, the primer mix provided in this kit can be used for amplification; if a "short adapter" or "small Y adapter" is used for DNA ligation, index primers are needed for amplification.
3.3 Amplification cycle numbers should be strictly controlled. Insufficient amplification may lead to low library yield; Over-amplification may introduce increased bias, errors, duplicated read, chimeric products, and accumulation of expansion mutations. Table 2 lists the recommended cycle numbers for PCR amplification.
Table 2 The recommended number of cycles to generate RNA library*
|
Input Total RNA |
Number of cycles |
|
|
Non-stranded |
Stranded |
|
|
10 ng |
15 |
15 |
|
100 ng |
14 |
14 |
|
500 ng |
12 |
13 |
|
1 μg |
11 |
12 |
Note:*The yield of the library is not only related to the input quantity and the number of amplification cycles but also affected by the quality of samples, fragmentation conditions and sorting conditions. In the process of library construction, choose the most appropriate conditions according to the actual situation.
4 Bead-based DNA Cleanup and Size Selection
4.1 There are multiple steps in the library construction process that require DNA purification magnetic beads. We recommend Hieff NGS™ DNA Selection Beads (Yeasen Cat#12601) or AMPure® XP magnetic beads (Beckman Cat#A63880) for DNA purification and size selection.
4.2 The magnetic beads should be equilibrated at room temperature prior to use, otherwise the yield will decrease and the size-selecting effect will be affected.
4.3 The magnetic beads should be mixed well by vortex or pipetting prior to use.
4.4 Do not aspirate the beads when transferring the supernatant, even trace amounts of the beads may impact the following reactions.
4.5 The 80% ethanol should be freshly prepared, otherwise it will affect the recovery efficiency.
4.6 The magnetic beads should be dried at room temperature before eluting the product. Insufficient dryness will easily cause ethanol residual to affect subsequent reactions; excessive dryness will cause the magnetic beads to crack and reduce the purification yield. Normally, drying at room temperature for 3-5 minutes is enough to allow the beads to fully dry.
4.7 If needed, the purified or size-selected DNA samples eluted in TE buffer can be stored at 4°C for 1-2 weeks or at -20°C for a month.
5 Library Quality Analysis
5.1 Normally, the quality of the constructed library can be evaluated by length distribution and concentration detection.
5.2 Library concentration detection: methods based on double-stranded DNA fluorescent dyes, such as Qubit®, PicoGreen®, etc.; absolute quantification based on qPCR.
5.3 Methods based on spectral detection, such as NanoDrop®, etc., is not applicable to library concentration detection.
5.4 qPCR is recommended for library concentration detection: Through Qubit®, PicoGreen® and other methods based on double-stranded DNA fluorescent dyes, it cannot effectively distinguish between products ligated to adapters at one end, products not ligated to adapters at both ends, and other incomplete double-strand products. Absolute quantification of qPCR is based on the principle of PCR amplification, which only quantifies the complete library of the adapter at both ends of the sample (the library that can be sequenced), excluding the interference of non-sequencing libraries that are not ligated to the adapter at either single-ended or double-ended ends.
5.5 Library length distribution detection can be performed by Agilent Bioanalyzer 2100 and other equipment based on the principle of capillary electrophoresis or microfluidics.
Citations & References:
[1] Deciphering the gut microbiome of grass carp through multi-omics approach. M Li, H Liang, H Yang, Q Ding, R Xia, J Chen, W Zhou… - Microbiome, 2024 IF:19.4
[2] Pooled CRISPR Screening Identifies P-Bodies as Repressors of Cancer Epithelial-Mesenchymal Transition. L Fang, L Zhang, M Wang, Y He, J Yang, Z Huang… - Cancer Research, 2024 IF:12.7
[3] Klotho-derived peptide 1 inhibits cellular senescence in the fibrotic kidney by restoring Klotho expression via posttranscriptional regulation. X Zhang, L Li, H Tan, X Hong, Q Yuan, FF Hou… - Theranostics, 2024 IF:12.4
[4] Exoskeleton Partial‐Coated Stem Cells for Infarcted Myocardium Restoring. H He, Y Yuan, Y Wu, J Lu, X Yang, K Lu… - Advanced Materials, 2023 IF: 29.4
[5] Genomic innovation and regulatory rewiring during evolution of the cotton genus Gossypium. M Wang, J Li, Z Qi, Y Long, L Pei, X Huang… - Nature Genetics, 2022 IF:41.379
[6] MTMR3 risk alleles enhance Toll Like Receptor 9-induced IgA immunity in IgA nephropathy. Y Wang, T Gan, S Qu, L Xu, Y Hu, L Liu, S Shi, J Lv… - Kidney International, 2023 IF:19.6
[7] Split complementation of base editors to minimize off-target edits. X Xiong, K Liu, Z Li, FN Xia, XM Ruan, X He, JF Li - Nature Plants, 2023 IF:18.6
[8] Engineered bacterial outer membrane vesicles encapsulating oncolytic adenoviruses enhance the efficacy of cancer virotherapy by augmenting tumor cell autophagy. W Ban, M Sun, H Huang, W Huang, S Pan… - Nature Communications, 2023 IF:16.6
[9] Cancer Cell Resistance to IFNγ Can Occur via Enhanced Double-Strand Break Repair Pathway Activity. T Han, X Wang, S Shi, W Zhang, J Wang, Q Wu… - Cancer Immunology Research, 2023 IF: 12.0
[10] Combination therapy based on dual-target biomimetic nano-delivery system for overcoming cisplatin resistance in hepatocellular carcinoma. Y Huang, Q Kou, Y Su, L Lu, X Li, H Jiang… - Journal of Nanobiotechnology, 2023 IF: 10.9
[11] PIAS3 promotes ferroptosis by regulating TXNIP via TGF-β signaling pathway in hepatocellular carcinoma. W Bao, J Wang, K Fan, Y Gao, J Chen - Pharmacological Research, 2023 IF:9.3
[12] Identification of RNA-Binding Protein Targets with HyperTRIBE in Saccharomyces cerevisiae. W Piao, C Li, P Sun, M Yang, Y Ding, W Song… - International Journal of molecular Science, 2023 IF:5.6
[13] Microbiota regulates life-cycle transition and nematocyte dynamics in jellyfish. S Peng, L Ye, Y Li, F Wang, T Sun, L Wang, W Hao… - Iscience, 2023 IF: 5.08
[14] Transcriptome and miRNAs Profiles Reveal Regulatory Network and Key Regulators of Secondary Xylem Formation in “84K” Poplar. H Wang, P Zhao, Y He, Y Su, X Zhou… - International Journal of molecular Science, 2023 IF:5.6
Catalog No.:*
Name*
phone Number:*
Lot:*
Email*
Country:*
Company/Institute:*

