In vitro nucleic acid, amplification is the basis of molecular biology research, in which polymerase chain reaction (PCR) is the first and most widely used nucleic acid amplification technology. Conventional PCR technology relies on specific equipment and has many shortcomings, such as various factors influencing amplification, high price, and long time-consuming, which limits its application in primary and clinical field detection. In contrast to conventional PCR, Isothermal Amplification Technology (IAT) only needs a constant temperature device (such as a water bath) to achieve the purpose of efficiently and rapidly amplifying nucleic acid via enzymes with different activities and specific primers at a constant temperature, which is more suitable for rapid clinical diagnosis of pathogens. So what exactly is isothermal amplification technology, and what are the differences compared with conventional PCR technology?
1. What is isothermal amplification technology?
2. What are the types of isothermal amplification techniques?
3. What is the core enzyme of LAMP application?
4. Recommended products related to isothermal amplification
5. Regarding reading
1. What is isothermal amplification technology?
In recent years, with the rapid development of molecular biology technology, nucleic acid detection-based diagnostic methods have been established and widely used in the laboratory detection of human diseases. Isothermal amplification technology is an in vitro nucleic acid amplification technology. The reaction process is always maintained at a constant temperature, and the purpose of rapid nucleic acid amplification is achieved by adding enzymes with different activities and their respective specific primers. Compared with other nucleic acid amplification techniques, isothermal amplification has the advantages of rapidity, high efficiency, and specificity, and does not require special equipment. Therefore, it has been considered by many scholars as a detection method that may be comparable to PCR as soon as it appeared. Currently, conventional PCR is still the most popular amplification technology used to amplify and detect low-abundance nucleic acids and has been widely used in various fields. But it requires large and expensive thermal cyclers, which greatly limits the use of PCR in resource-constrained settings and for point-of-care analysis. Compared with traditional PCR, isothermal amplification can be performed at one reaction temperature and under simple conditions (such as in a water bath), so that rapid and efficient amplification can be achieved without relying on thermal cycling. Since the early 1990s, dozens of isothermal nucleic acid amplification techniques employing various amplification mechanisms have been developed, and most of these methods have surprisingly high sensitivity for the detection of nucleic acids. Thousands of studies related to the isothermal amplification of nucleic acids have been published to date.
Table 1. Comparison of conventional PCR technology and isothermal amplification technology
|
Characteristics |
PCR |
LAT |
|
Amplification |
3 steps: 95°C denaturation; ~60°C primer annealing; 72°C polymerization extension |
Constant temperature of 60-65” C |
|
Denaturation |
DNA double strands melt at high temperature |
Melting is done directly by strand displacement active DNA polymerase |
|
Reaction time |
≥90 min |
15-30 min |
|
Instrument |
Thermal circulators are required |
No dedicated thermal cycler required |
|
Result |
Nucleic acid fragments can be visualized by gel electraphoresis |
Results can be directly observed colorimetric/visual turbidimetry |
|
Sensitivity |
Nanogram level targets can be detected |
Femtogram level targets can be detected |
|
Advantages |
Mature technology; large flux; high sensitivity and specificity |
Fast; low equipment requirements, simple procedure, easy to realize automatic and integration |
|
Disadvantages |
The operation is complicated and the amplification time is long: the precision and reliability of the instrument are required to be high; there are many factors affecting the amplification |
Flux is low; Primer design is difficult: Reagent prices are high |
2. What are the types of isothermal amplification techniques?
Isothermal nucleic acid amplification techniques can be divided into many categories according to different amplification principles, including Loop-Mediated Isothermal Amplification (LAMP), Cross-Primer Amplification (CPA), Strand Displacement Amplification (SDA), Recombinase Polymerase Amplification (RPA), Nucleic Acid Sequence Based amplification (NASBA), Rolling Loop Amplification (RCA) and Helicase-Dependent Amplification (HAD), etc.
2.1 LAMP
LAMP is carried out at 60-65 °C and requires 4 primers and a DNA polymerase from Bacillus stearothermophilus with strand displacement function. The outer primers are similar to the PCR primers, and the inner primers contain two sequences. Specific process: the internal primer binds to the target gene and is extended into a double strand under the action of BstDNA polymerase. The outer primer binds to the 5' end of the double-stranded DNA, forming a circular structure at one end. The other end goes through the same process to form a dumbbell-shaped structure with rings at both ends. Single-stranded DNA with a dumbbell-shaped structure has the dual functions of template and primer and is extended under the catalysis of Bst polymerase. Inner primers can also bind to the loop structure and be extended by enzymes.
2.2 CPA
CPA is carried out at about 63 ℃, relying on Bst DNA polymerase, betaine, and cross primers. According to the number of cross primers, it can be divided into double cross primer amplification and single cross primer amplification. Double crossover primer amplification uses two crossover primers and two stripper primers. The two cross primers are complementary to the template strand and then extended, and then the stripping primer strips the newly synthesized single strand under the action of Bst DNA polymerase, and the last two cross primers use the nascent single strand as Template synthesis of a large number of target fragments. Single cross-primer amplification uses one cross primer, two stripper primers, and two normal primers. First, the cross primer combines with the template strand and extends into a double strand, and the stripping primer separates the new strand from the template under the action of Bst DNA polymerase; then the common primer uses the new strand as a template to synthesize two single-stranded DNAs of different lengths; Finally, the two single strands are used as templates, and the cross primer and common primer are used as primer pairs to form an amplification cycle.
2.3 SDAs
The reaction temperature of SDA is about 37 ℃. The reaction requires a restriction endonuclease, a strand-displacing DNA polymerase, two pairs of primers, and one pair of primers (P1 and P2) containing an endonuclease recognition sequence. At the beginning of the reaction, P1 and P2 are complementary to the template strand, which is extended into a double strand under the catalysis of the polymerase, and then the endonuclease recognizes the enzyme cleavage sites at both ends of the double strand and cuts to form a sticky end. The second pair of primers bind to the end of the template strand, and under the action of the polymerase, a new strand is synthesized and a single strand is replaced.
2.4 RPA
The reaction temperature of RPA is 37-42 ℃, and the reaction system includes a pair of primers and three key enzymes: recombinase capable of binding to oligonucleotide primers, single-strand DNA-binding protein, and strand-displacing DNA polymerase. At the beginning of the reaction, the primer combines with the recombinase to form a primer-recombinase complex, and complementarily binds to the corresponding site on the template strand, resulting in a change in the conformation of the double-stranded DNA, which is extended under the catalysis of the DNA polymerase with strand displacement properties to form a complete double chain. During the reaction, the single-strand binding protein binds to the free single-strand and maintains its stability.
2.5 NASBA
NASBA technology is an isothermal amplification method for detecting RNA, usually performed at around 42 °C, and requires avian myeloblastosis virus (AMV) reverse transcriptase, RNase H, T7 RNA polymerase, and a pair of primers to complete. Its forward primer contains the complementary sequence of the T7 promoter. During the reaction process, the forward primer combines with the RNA strand, and is catalyzed by the AMV enzyme to form a DNA-RNA double strand; RNase H digests the RNA in the hybrid double strand, and retains the DNA single strand; under the action of the reverse primer and AMV enzyme, it forms a DNA-RNA double strand containing T7 The DNA double strand of the promoter sequence; under the action of T7 RNA polymerase, the transcription process is completed to produce a large amount of target RNA.
2.6 RCA
RCA borrows from the way circular DNA replicates in nature. The required enzyme is phi29 DNA polymerase, which is carried out at about 37 °C. The process of ordinary RCA is that the primer is combined with the circular DNA template and then extended to generate a DNA single strand containing a large number of target genes.
2.7 HDA
HDA simulates the DNA semi-conservative replication process in vivo. The reaction is carried out at about 37 °C and depends on helicase, SSB, DNA polymerase, and a pair of primers. The process is as follows: DNA double strands are unwrapped under the action of helicase, SSB combines with single-strand DNA to keep it stable; at the same time, primers combine with single strands to form double strands under the catalysis of polymerase; newly synthesized DNA double strands serve as The template enters a new round of amplification.
3. What is the core enzyme of LAMP application?
LAMP is the most common and widely used isothermal amplification technology developed by Japanese scholar Notomi in 2000. The technical feature of LAMP is to design four primers for six regions of the target genes, and Bst DNA polymerase is used to perform amplification reaction at a constant temperature of 60~65℃ and achieve 109-10 fold amplification within 15-60 min.
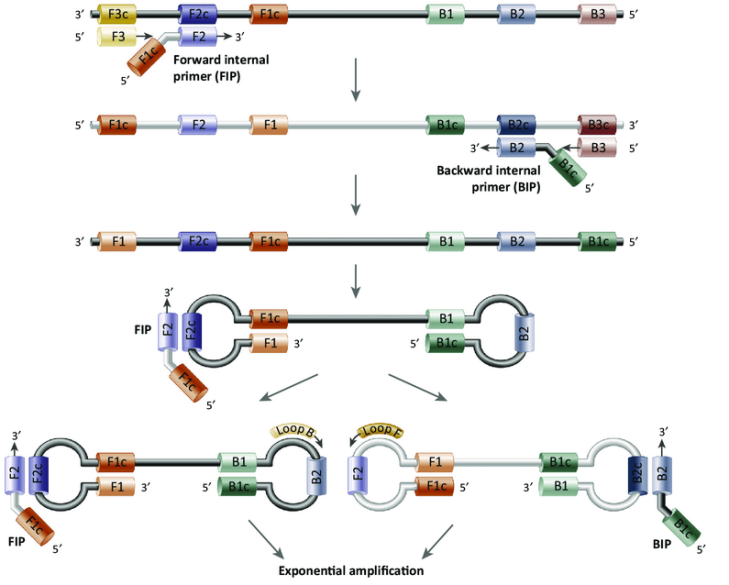
Figure 1. Schematic diagram of Loop-Mediated Isothermal Amplification (Alhassan A, et al. 2015, Trends in parasitology)
Bst DNA polymerase is the core enzyme of LAMP isothermal amplification technology. It has strong thermal stability, strand displacement activity, and polymerase activity. It can rapidly, efficiently, and specifically, amplify templates under constant temperature conditions and complete the entire LAMP experiment within 1 hour.
Yeasen Biotech the newly upgraded Hieff™ Bst Plus DNA Polymerase (Cat#14402ES, 14403ES) is obtained by expressing and purifying the DNA polymerase gene of Thermophilic Geobacillus sp lacking the 5′→3′ exonuclease domain in E.coli. It has 5′→3′ DNA polymerase activity and strong strand displacement ability. Compared with the wild type, the enzyme's sensitivity, amplification efficiency, and stability all have been greatly improved, and the dUTP tolerance has been increased. It can be widely used in the rapid detection of pathogens based on isothermal amplification technology, single nucleotide polymorphism sequencing, rapid sequencing of GC-rich or ng-level DNA templates, and isothermal strand displacement reactions during the next-generation sequencing libraries construction.
4. Recommended products related to isothermal amplification
The products that Yeasen provided are as follows.
Table 2. Product Selection Guide
|
Experiment Type |
Application scenarios |
Product Positioning |
Product name |
Cat# |
|
LAMP |
DNA Pathogen Detection |
Bst with high amplification capacity and dUTP tolerance |
14402ES | |
|
Hieff™ Bst Plus DNA Polymerase (2,000 U/μL) 👍(Inquire) |
14403ES |
|||
|
Heat-labile UDG |
Uracil DNA Glycosylase (UDG/UNG), heat-labile, 1 U/μL (Inquire) |
10303ES |
||
|
High Purity dNTP Mix |
10125ES |
|||
|
RT-LAMP |
RNA pathogen detection |
Strand displacement isothermal amplification |
Hieff™ Bst DNA polymerase (40 U/μL, Glycerol-free) (Inquire) |
12910ES |
|
Bst with high amplification capacity and dUTP tolerance |
14402ES |
|||
|
Hieff™ Bst Plus DNA Polymerase (2,000 U/μL) 👍 (Inquire) |
14403ES |
|||
|
Reverse transcriptase with high amplification capacity |
Hifair™ III Reverse Transcriptase third generation thermostable reverse transcriptase (Inquire) |
11111ES |
||
|
Reverse transcriptase with high amplification capacity in glycerol-free version |
Hifair™ III Reverse Transcriptase, 600 U/μl, Glycerol-free third-generation thermostable reverse transcriptase (glycerol-free version) (Inquire) |
11297ES |
||
|
Heat-labile UDG |
Uracil DNA Glycosylase (UDG/UNG), heat-labile, 1 U/μL (Inquire) |
10303ES |
||
|
High Purity dNTP Mix |
10125ES |
|||
|
Highly specific RNase inhibitor |
10603ES |
5. Regarding reading
High-quality isothermal amplification raw materials ——Making RT-LAMP more sensitive and faster!
References:
1. Alhassan A, Li Z, Poole C B, et al. Expanding the MDx toolbox for filarial diagnosis and surveillance[J]. Trends in parasitology, 2015, 31(8): 391-400.