Introduction Quality Control Strategies for Biological Products
01
Concept and regulatory significance of biological products
According to the Chinese Pharmacopoeia, biological products are medicines made from microorganisms, cells, tissues and body fluids of animal or human origin, etc., and made by applying traditional technology or modern biotechnology, and used for the prevention, treatment and diagnosis of human diseases. Among them, biological products for human use include bacterial vaccines, viral vaccines, antitoxins and antisera, blood products, cytokines, growth factors, enzymes, in-vivo and in-vitro diagnostic products, as well as other biologically active preparations.
Biological products have gained rapid development due to their stronger specificity, ability to bind to molecular targets, good efficacy and fewer side effects. However, biologics are characterized by diverse components, complex structures and different production processes, which make them more susceptible to contamination and impurities in the preparation process.
Common contaminants are carried exogenous substances such as pathogenic microorganisms carried by biological raw materials (eg. bacteria, mycoplasma, mycobacteria, endogenous viruses and externally contaminated viruses). Impurities are generally categorized into product-related impurities and process-related impurities. Product-related impurities include aggregates, degradation products, charge isomers, hydrophobic variants, inactive viral particles, polysaccharides not in the intended range, and virus-like particles with incomplete packaging. Process-related impurities include cellular matrix (host cell proteins, host cell DNA), cell cultures (inducers, antibiotics, or media components), or downstream process residues. These impurities and contaminants, etc. will not only affect the efficacy of the biologic drug, but may also have safety concerns, so the drug development and manufacturing process requires strict quality control.
02
Market Size of QC Testing
With the rapid development of the global biopharmaceutical industry, the QC market has also seen rapid growth. On the one hand, the continuous innovation of drugs has raised the demand for biosafety QC testing: from the early days of chemical drugs, recombinant protein drugs, traditional vaccines to later antibody drugs, gene cell therapy drugs, mRNA vaccines, small nucleic acid drugs, stem cell products and other drugs, the innovation of drugs has brought about the complexity of the requirements for biosafety testing. On the other hand, the development of the biopharmaceutical industry also puts forward high requirements for quality control: the growth of the market scale of biopharmaceuticals and the increase of R&D investment promote the demand for safety and quality control in the R&D process; the founders of innovative pharmaceutical companies return home from overseas, and the importance of quality control and safety and the requirements of quality control and safety are raised, and the increase in the pipeline of innovative drugs in China, the United States, and China-EU dual-reporting is a good example of how safety and quality control requirements are brought into line with the international standard; and the emerging of new therapeutic technologies, such as The emergence of new therapeutic technologies, such as gene therapy, cell therapy and nucleic acid drug development, and other emerging therapies and technologies have put forward brand new requirements for quality control and safety.
Although the quality control market size is growing, but the quality control testing of biological products is currently less attention by the capital market, so the relevant industry research reports are relatively limited, the following is the current access to the quality control related to China's market size:
China Cell Quality Assay and Biosafety Assessment Market Size and Forecasts
Unit: 100 million RMB
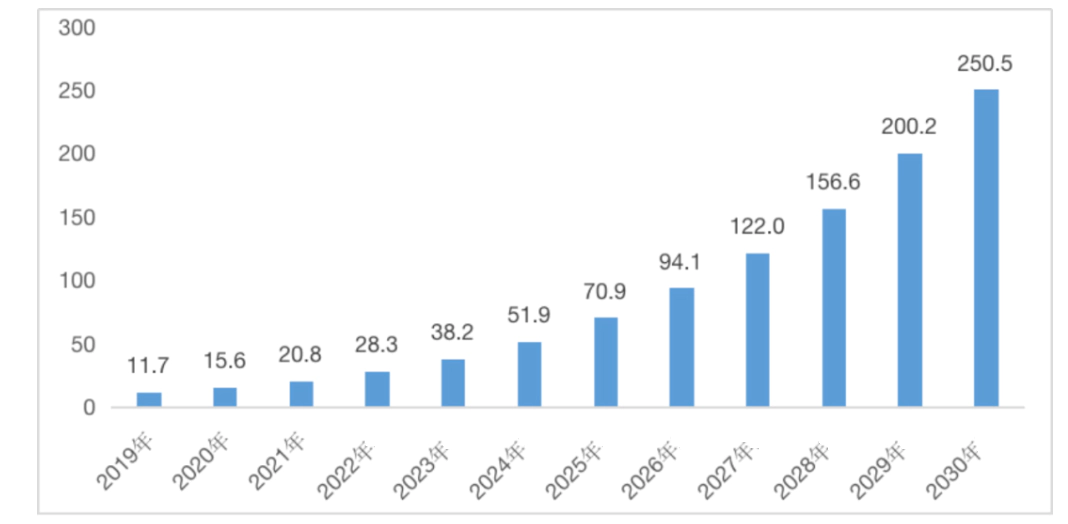
Source: Frost & Sullivan analysis.
03
Yeasen Biotechnology Quality Control Analytical Solutions
Impurity residues and contaminants involved in the production process of biologics have a significant impact on the safety and efficacy of the final product and are strictly regulated by the drug regulatory authorities of various countries. Therefore, Yeasen Biotechnology has independently developed a series of biologics quality and safety control products for the detection of host cell DNA residues, nuclease residues, replicative lentivirus, viral vector copy number and mycoplasma contamination.

04
Yeasen Biotechnology R&D and production platform for quality control assay reagents
R&D Platform
Based on the platform of bidirectional molecular enzyme design and directed evolution and the platform of protein high-density fermentation and ultra-clean purification, we have set up the R&D platform of key raw materials for molecular diagnostic reagents and an independent molecular and immunoassay product development laboratory, which are equipped with advanced R&D instruments and equipments.
Production platforms
Constructed and managed in strict accordance with ISO13485 quality system standards, we have independent clean production workshops to meet the quantitative and qualitative requirements of different products. Layer by layer, each step of the release has gone through a number of strict and high standard quality acceptance.

Yeasen Biotechnology R&D and production workshop for quality control assay reagents