With the advancement of stem cell therapy and organoid-based drug development, basement membrane matrix plays a pivotal role as a nutrient and support carrier for stem cell cultures and organoids, 3D cell cultures, and other applications including angiogenesis, in vivo tumorigenesis experiments, etc. Ceturegel™ basement membrane Extracts are extracted from Engelbreth-Holm-Swarm (EHS) mouse tumors rich in extracellular matrix proteins including laminin, type IV collagen, nestin, etc. IGF, FGF, and other growth factors. At room temperature, the Ceturegel™ basement membrane matrix polymerizes to form a biologically active three-dimensional matrix. It can simulate the structure, composition, physical properties, and functions of the cell basement membrane in vivo, which is beneficial to the culture and differentiation of cells in vitro and is a good matrigel alternative.
1. What is Ceturegel™ basement membrane matrix?
2. What is the role of Ceturegel™ basement membrane matrix?
3. What are the characteristics of Ceturegel™ basement membrane matrix?
4. The popular application of Ceturegel™ basement membrane matrix
5. FAQs
6. The selection guide of Ceturegel™ basement membrane matrix from Yeasen
1. What is Ceturegel™ basement membrane matrix?
The matrix adjacent to endothelial cells, epithelial cells, muscle, and neuronal cells forms a continuous, layered extracellular matrix called the basement membrane. The basement membrane degenerates and regenerates during development and wound healing. It not only supports cells and cell layers but also plays an important role in the formation of tissues by affecting cell adhesion, migration, proliferation, and differentiation, which are functions of the basement membrane. Therefore, it can be said that the basement membrane is the main barrier to the invasion of metastatic tumor cells.
Figure 1. The Ceturegel™ basement membrane matrix
The Ceturegel™ basement membrane matrix developed and produced by YEASEN does not contain LDEV (Lactate Dehydrogenase Enhancing Virus) and has ultra-low endotoxin content. And after mycoplasma detection to ensure no mycoplasma contamination, including different types such as basic concentration, high concentration, and low growth factor.
2. What is the role of Ceturegel™ basement membrane matrix?
Ceturegel™ basement membrane matrix can be used to prepare basement membrane matrices of various requirements. It can be used for cell signaling studies, such as the study of the role of growth factors in the formation of renal tubules by mouse kidney stem cells, the gene expression study of mouse mammary epithelial stem cells, and the tumor invasiveness experiment of Transwell. At the same time, it can be used for the study of cell morphology, biochemical function, migration, infection, and gene expression. Ceturegel™ basement membrane matrix can effectively help the attachment and differentiation of epithelial cells and other types of cells, including nerve cells, stem cells, mammalian epithelial cells, melanoma cells, vascular endothelial cells, thyroid cells, and hair follicle cells. At the same time, Ceturegel™ basement membrane matrix also affects the protein expression level of murine mammary epithelial cells and supports peripheral nerve regeneration.

Figure 2. The main application directions of Ceturegel™ basement membrane matrix
Cell migration and invasion in detail: Cell migration, also known as cell crawling, movement, or movement, refers to the movement of cells after they receive a migration signal or feel a gradient of certain substances. Cell migration is an alternating process of extension of pseudopodia in the cell head, establishment of new adhesions, and retraction of the tail of the cell body. Cell migration is one of the basic functions of normal cells, and it is also a physiological process of normal growth and development of the body. As a ubiquitous form of movement of living cells, it can participate in a variety of collective physiological and pathological processes. Such as embryonic development, angiogenesis, wound healing, immune response, inflammatory response, atherosclerosis, cancer metastasis, etc. Whereas, cell invasion refers to the ability of cells to migrate from one area to another through the extracellular matrix. Cell invasion is the response of normal cells and cancer cells to chemical and mechanical stimuli. Cell invasion often occurs in the processes of wound healing, angiogenesis, inflammation, tumor cell metastasis, and abnormal infiltration of tissues.
3. What are the characteristics of Ceturegel™ basement membrane matrix?
High safety: no LDEV (lactate dehydrogenase increased virus)
Concentration diversity: the concentration range is between 8~20 mg/ml
Good batch stability: strict production quality inspection process to ensure stable performance between batches
Low endotoxin: endotoxin content <8 EU/ml
Contamination detection: no mycoplasma, bacteria, and fungi residues have been detected
High single batch output: single batch output is above the 50L level
Compatibility: Compatible with any type of cell culture medium
4. The popular application of Ceturegel™ basement membrane matrix
4.1 Migration and invasion assay
The experimental method to detect the ability of cell migration and invasion is the Transwell experiment, and Transwell is also called the perforation experiment. The cell suspension is added to the chamber first because the chamber has dense pores. The chambers were then placed in a 24-well plate to which a complete medium was added. Cells deformed and passed through holes in the chamber to the outside of the more nutrient-rich chamber, where they stuck to the outside. By staining and counting the cells outside the chamber, the migration and invasion ability of the cells can be judged. The principle of Transwell is to put the small chamber in the culture plate, the small chamber is called the upper chamber, and the culture plate is called the lower chamber. The upper and lower layers of culture fluid are separated by a polycarbonate membrane, the upper layer of culture fluid is added to the upper chamber, and the lower layer of culture fluid is added to the lower chamber. The cells are in the upper chamber, and the composition of the lower medium will affect the cells in the upper chamber due to the permeability of the membrane. Furthermore, the effects of the components in the lower medium on cell growth and movement were investigated.
Specific operations of Ceturegel™ basement membrane matrix in migration and invasion assays: The diluted Ceturegel™ basement membrane matrix was added to the upper chamber of the Transwell, and the cells were plated and incubated in a 37°C, 5% CO2 incubator for 24 hours, fixed in 4% paraformaldehyde, and stained with 0.1% crystal violet staining solution. Cells were observed and counted under an inverted phase contrast microscope.
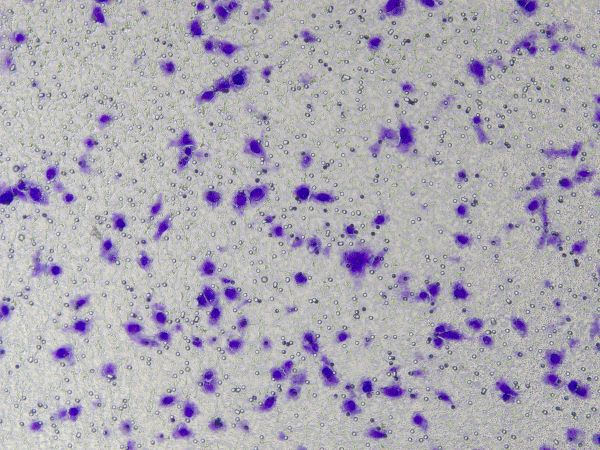
Figure 3. Results of crystal violet staining after cell invasion
4.2 Angiogenesis
1) One day before the experiment, take out the Ceturegel™ Matrigel from the freezer and place it in a 4°C refrigerator overnight to thaw while pre-cooling the used consumables.
2) Always keep Ceturegel™ Matrigel in an ice box before the experiment. Open the sterile packaging of angiogenic slides and remove the slides.
3) Add 10 μl Ceturegel™ Matrigel to each well. Note that the pipette tip should be perpendicular to the top of the inner hole when adding Ceturegel™ Matrigel to prevent the Matrigel from flowing through the upper hole and leaving a glue residue.
4) First cover the slide, prepare a 10 cm petri dish, and put paper towels soaked in water to make a wet box.
5) Put the slides into the petri dish and cover the petri dish. Put it in a CO2 incubator, let it stand for about 30 minutes, wait for the gel to coagulate, and prepare the cell suspension at the same time.
6) Prepare the digested cells into a cell suspension with a density of 2*105 cells/ml and mix thoroughly.
7) Remove the glass slide containing the blood vessel that has solidified into a gel. Add 50 μl of the cell suspension to each well, taking care to keep the pipette tip vertically above the upper well and not touch the gel in the lower well.
8) Add the cell culture medium, close the lid, and let it stand. After some time, all cells will sink to the surface of the Matrigel.
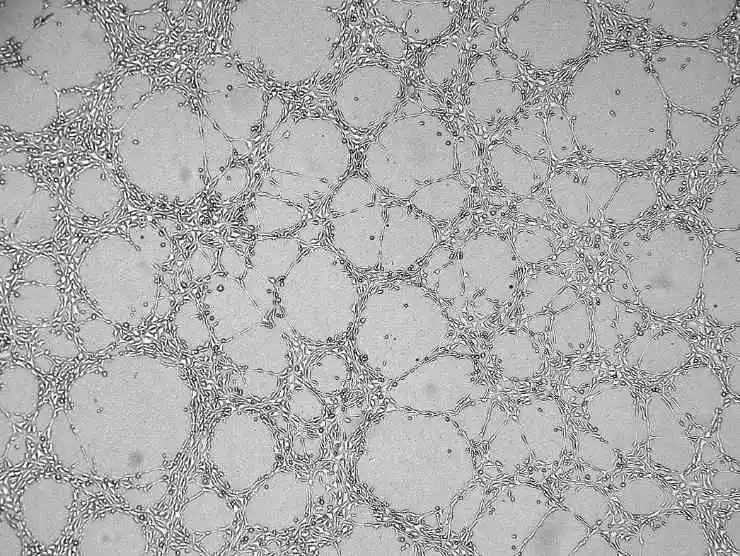
Figure 4. Angiogenesis results graph
Immunofluorescence staining
1) Carefully remove the medium from the wells without touching the glue or cell network. Dilute calcein in serum-free medium to a final concentration of 6–8 µg/ml. Add cell staining solution to completely submerge the cells, and incubate at room temperature for 30-40 minutes in the dark.
2) Wash three times with PBS. Note that PBS should be slowly added to the upper wells to avoid impacting the cells. Fluorescence observation using Ex=485 nm, Em=529 nm wavelength
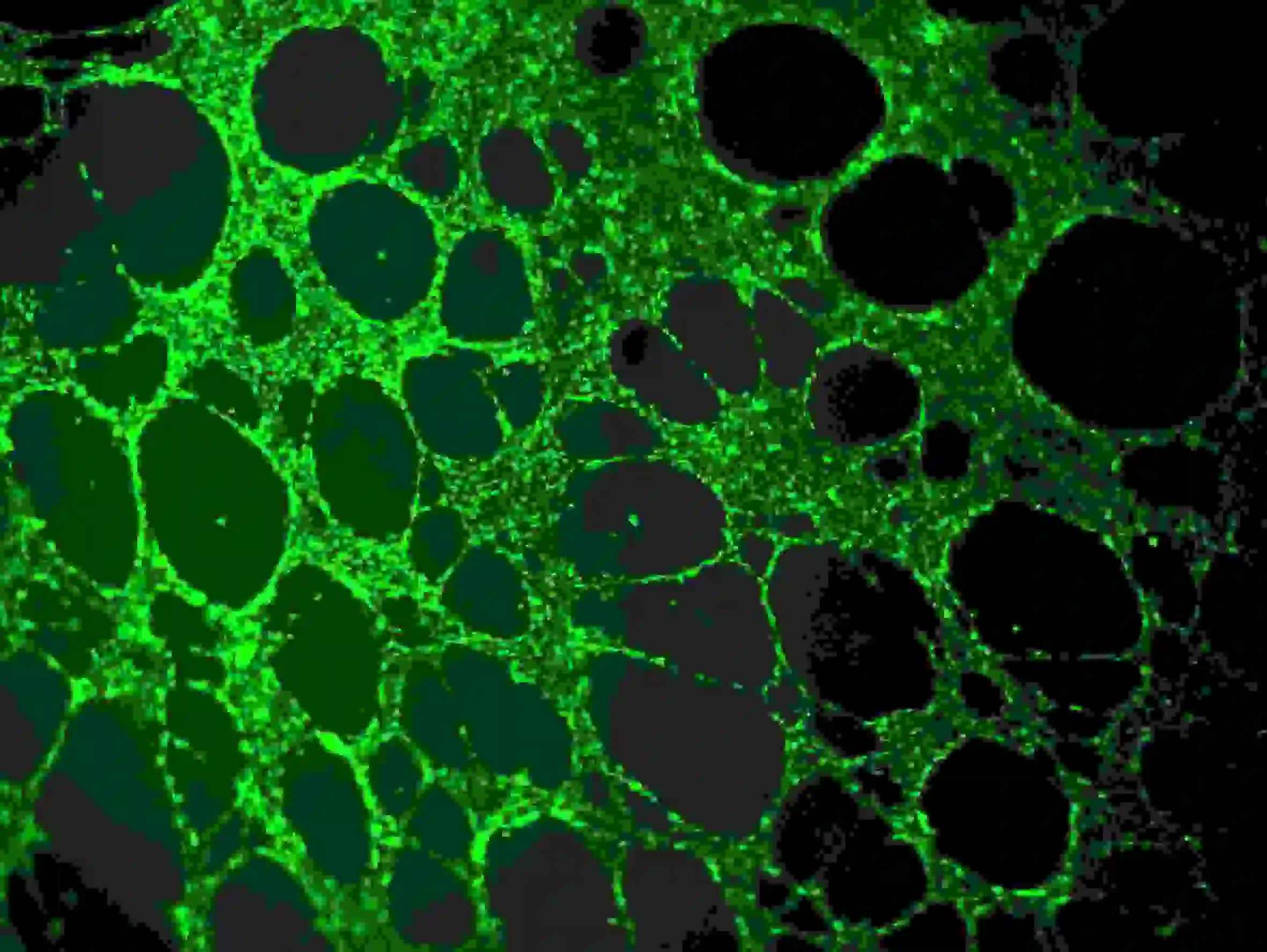
Figure 5. Immunofluorescence staining of blood vessels
4.3 3D cell culture
Unlike traditional cell culture, 3D cell culture reproduces the in vivo environment of cells. Even simple spheroid models can compensate for the shortcomings of monolayer cultures. These structures can form gradients of oxygen, nutrients, metabolites, and soluble signals, which in turn form diverse cell populations. 3D cell culture technology can better simulate the natural environment in which cells live in organisms, making the interactions between cells and biochemical and physiological responses more realistic. In a 3D environment, cells' responses to endogenous and exogenous stimuli more closely resemble their in vivo responses.
The specific operation of Ceturegel™ basement membrane matrix in 3D cell culture is as follows: Gently mix the Ceturegel™ basement membrane matrix with the adjusted concentration of single-cell HepG2 suspension 1:1, and add 50 μl of the above-mixed single-cell suspension to the pre-cooled 24-well plate with a pre-cooled pipette tip to form Arch-shaped cell droplets were cultured in a 37°C, 5% CO2 incubator, observed and photographed every day.
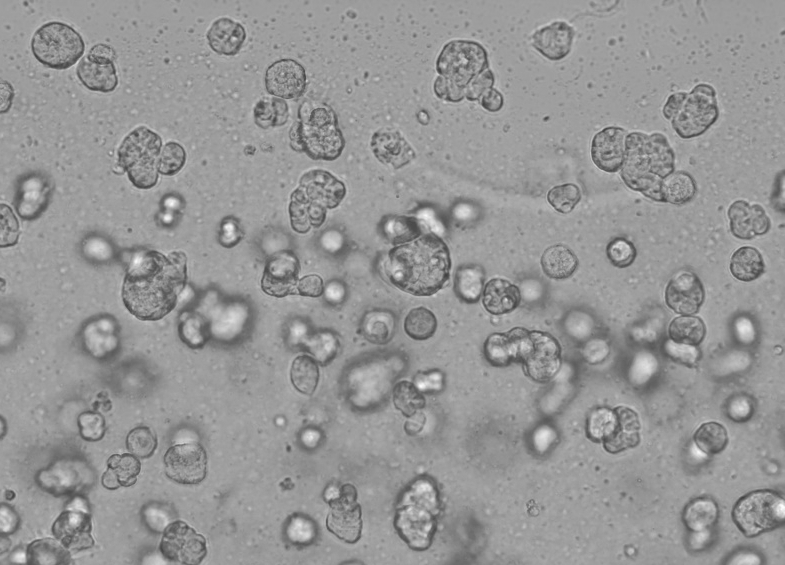
Figure 6. 3D cell culture results
Table 1. 3D Cell Culture Ceturegel™ basement membrane matrix Use Reference:
| Culture plate (dish) type | Cell culture area (cm2) | Usage metering (concentration ≥ 3 mg/mL)* |
|---|---|---|
| 6-well plate | 9.6 | 200 μL/cm2 |
| 12-well plate | 4.5 | 180 μL/cm2 |
| 24-well plate | 2.0 | 180 μL/cm2 |
| 96-well plate | 0.32 | 160 μL/cm2 |
| 35mm dish | 11.78 | 200 μL/cm2 |
| 100mm dish | 58.95 | 200 μL/cm2 |
Note: Different batches of Ceturegel™ basement membrane matrix have a certain concentration difference, the recommended dosage is for reference only
4.4 Tumor formation experiment in vivo
Taking the subcutaneous tumorigenesis experiment of HepG2 cells in nude mice as an example, Ceturegel™ basement membrane matrix and cell suspension were used for 1:1 dilution, and BALB/c-nu female mice aged 4-5 weeks were inoculated subcutaneously. The experimental process is as follows:
♦ Prepare HepG2 cells with logarithmic growth and cell density of about 80-90%, and change the fresh medium the night before collecting cells.
♦ The cells are digested by trypsin. When the cells become round and do not leave the culture dish, the trypsin is removed, the serum-free medium is added to make the cell suspension, centrifuged and cleaned once, and the final concentration is 5 × 107 cells/mL.
♦ Dilute the cell suspension and Ceturegel™ basement membrane matrix in 1:1 ratio at 4 ℃ to prepare a final concentration of 5 × 107 cells/mL.
♦ Grab a fixed nude mouse with the left hand, and inject it subcutaneously at the right shoulder of the nude mouse. During the inoculation, the needle is inserted subcutaneously a little deeper, about 1cm deep, to reduce the overflow of cell suspension from the needle eye after the injection.
The inoculation volume is 200 μ L。 (This process should be completed within half an hour as far as possible. On the way, cell suspension should be placed on ice to slow down cell apoptosis and prevent gel phenomenon).
♦ Put the nude mice back into the cage to continue feeding, and the tumor can be seen for about 1 week to 1 month. According to the experimental design, euthanize the nude mice when the tumor volume meets the requirements, and take photos.
Note: The control group is the suspension of the culture medium and cells, and the final density is the same as that of the matrix glue test group.
4.5 Organoid culture
Organoids are 3D multicellular tiny tissues differentiated from stem cells. Some properties of organs can be reproduced. Organoids are multicellular and exhibit a high degree of self-assembly, and are therefore better able to exhibit complex in vivo cellular responses and interactions than traditional 2D cultures. Stem cells and/or organ progenitor cells from normal or diseased tissue can be mixed with Ceturegel™ basement membrane matrix or collagen. Form kidney, thyroid, liver, brain, lung, intestine, prostate, and other micro-organs. For example, for researchers conducting genetic screens, Ceturegel™ basement membrane matrix substrates can be used as bioinks to enable precise localization and embedding of living cells/organoids in 3D bioprinting.
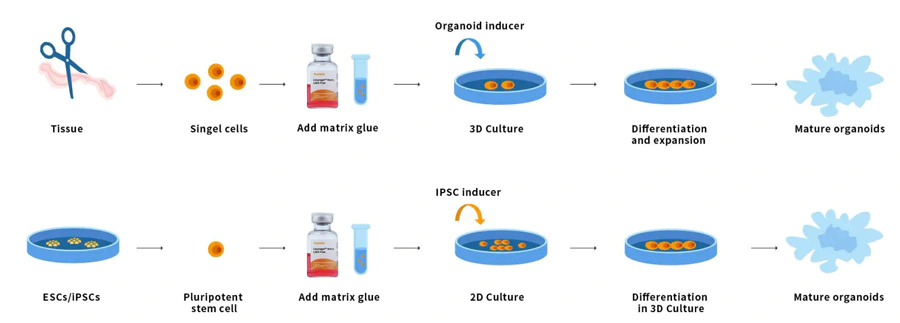
Figure 7. Organoid operation process
Mouse Small Intestinal Organoid Construction
Sample preparation: The mice were killed by cutting their necks, and the surface was sprayed with alcohol for sterilization. Cut out the intestinal tissue 3~15cm near the gastric end under the sterile environment, carefully remove the mesentery and fat outside the intestinal tract with tweezers, and put it into the DPBS solution containing 1% double antibody precooled at 4 ℃.
Sample cleaning: use a syringe to flush the intestinal tract 2-3 times, use surgical scissors to carefully cut the intestinal tract with the intestinal cavity facing up, and use a surgical blade to gently scrape off the intestinal villi on the surface of the intestinal cavity, and after the intestinal villi are scraped off (showing transparent tissue), place the intestinal tissue in a new culture dish containing DPBS for 2-3 times.
Initial treatment of samples: cut the washed small intestinal tissue into 2mm wide small pieces, and then transfer them into a new 50ml centrifuge tube. Wash them gently 3-5 times with DPBS to remove intestinal villus cells and floating fat tissue.
Sample digestion: add 10-15ml precooled DPBS containing 3-5mM EDTA to the cleaned small intestinal fragments for digestion, incubate at 4 ℃ for about 30min, and gently shake the centrifuge tube every 10min during this period.
After digestion, discard the supernatant of the EDTA digestion solution and gently rinse the tissues with new DPBS buffer solution 2-3 times to remove the remaining EDTA.
Add 10-15ml precooled DPBS containing 0.1% BSA into the small intestinal tissue fragments, blow and resuspend the tissue fragments repeatedly to separate the recess from the basal layer, then take a little suspension for microscopic examination. When a large number of recess-like structures are seen, stop blowing, and use 70% for the blown tissue suspension μM Filter screen to filter and collect the tissue suspension passing through the filter screen.
Repeat steps 5-6 twice and centrifuge at 1500rpm and 4 ℃ for 3min.
Formation of mixture: Ceturegel™ Matrix glue heavy suspension recess tissue precipitation, every 10 μL matrix glue suspension contains 200~600 recesses. After resuspension, the mixture is placed on ice and operated as soon as possible to avoid the matrix glue forming gel.
Note: dilution ratio of matrix glue ≥ 50% to ensure Ceturegel™ in the process of culture the stability of matrix adhesive structure.
Plant the mixed suspension at the center of the bottom of the 24-well plate, 30~50μL per well left and right to avoid the suspension contacting the side wall of the orifice plate.
Place the cultivated culture plate in the 37 ℃ carbon dioxide constant temperature incubator, and incubate it for about 30min until the matrix gel solidifies.
Wait for Ceturegel™ After the matrix glue is completely solidified, slowly add the prepared intestinal organ culture medium along the wall, 800μL per well.
Put the 24-well plate in the 37 ℃ carbon dioxide incubator for culture. Replace the fresh medium every 3 days and monitor the growth status of an organ like organs. Generally, the small intestine organ like organs of mice is formed within 5-7 days.
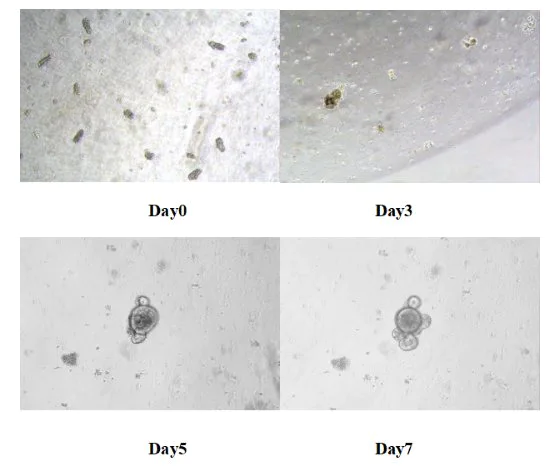
Figure 8. Results of in vitro culture of mouse small intestine-like organs
5. FAQs
1. What is the reason for the color difference (light yellow to dark red) of the obtained substrate?
For the Ceturegel™ basement membrane matrix containing phenol red, it is mainly caused by the interaction of phenol red and bicarbonate with CO2, but the color difference will be reduced after equilibration with 5% CO2. After freezing and thawing, shake the vial gently to disperse the Ceturegel™ basement membrane matrix evenly.
2. What matters should be paid attention to in the operation of Ceturegel™ basement membrane matrix?
All operations should be performed in a sterile environment, and a pre-cooled pipette should be used to ensure that the Ceturegel™ basement membrane matrix is homogenized.
3. How to freeze and store Ceturegel™ basement membrane matrix for use?
The frozen and thawed Ceturegel™ Matrix LDEV-Free Ceturegel™ basement membrane matrix can be distributed in multiple small tubes. All distributions should be in pre-cooled cryovials, which should be quickly frozen and stored to avoid multiple freezing and thawing. All items involved should be pre-cooled before use. Use pre-cooled pipettes, tips, and small tubes to handle Ceturegel™ basement membrane matrix.
6. The selection guide of Ceturegel™ basement membrane matrix from Yeasen
Different types of Ceturegel™ basement membrane matrix have different applications. Standard concentrations of Ceturegel™ basement membrane matrix can be used for polar cell cultures, such as epithelial cells. It can promote the differentiation of various cells and be used for tumor cell migration and invasion experiments. High concentrations of Ceturegel™ basement membrane matrix are widely used in vivo and can be used for tubule formation experiments. The main function of low growth factor (GFR) is to eliminate the interference of growth factors in the experiment, and it is suitable for studies with high requirements for basement membrane preparation. Ceturegel™ basement membrane matrix without phenol red can eliminate the interference of phenol red indicator and is suitable for color development experiments, such as colorimetry and fluorescence detection. Human embryonic stem cell culture grade Ceturegel™ basement membrane matrix is specially used for human embryonic stem cell culture, induced pluripotent feeder-free stem cell culture. Yeasen provides many types of Ceturegel™ basement membrane matrix, you can choose them based on your experiments.
Table 2. Ceturegel™Matrix Selection Guide
| Product type | Cat# | Product Name | Corresponding Corning Cat No. | Application direction |
| Basic concentration (8-12 mg/ml) | 40183ES | Ceturegel™Matrix LDEV-Free | 356234/354234 | Adapt to 2D and 3D culture, invasion, and migration experiments, and can also be used for in vivo tumorigenic experiments |
| 40184ES | Ceturegel™Matrix Phenol Red-Free,LDEV-Free | 356237 | Mainly used for color detection such as fluorescence detection experiments, etc | |
| High concentration (≥18mg/ml) | 40187ES | Ceturegel™Matrix High Concentration,LDEV-Free | 354248 | Mainly used in experiments such as angiogenesis, gel embolization, and in vivo tumor formation (for angiogenesis,it is recommended that the final concentration of Ceturegel™ basement membrane matrix should be ≥10mg/ml) |
| 40189ES | Ceturegel™Matrix High Concentration,GFR,LDEV-Free | 354263 | ||
| 40188ES | Ceturegel™Matrix High Concentration,Phenol Red-Free,LDEV-Free | 354262 | ||
| Growth factor reduction | 40185ES | Ceturegel™Matrix GFR,LDEV-Free | 354230 | Mainly to exclude the interference of growth factors on the experiment. Applied to related research on growth factors, signaling pathways, etc. |
| 40186ES | Ceturegel™Matrix GFR,Phenol Red-Free,LDEV-Free | 356231 | ||
| For stem cells | 40190ES | Ceturegel™Matrix hESC-Qualified,LDEV-Free | 354277 | Mainly used for stem cell culture such as hESC, iPSC, etc. |
| Organoid-specific | 40191ES | Ceturegel™Matrix for Organoid culture,Phenol Red-Free,LDEV-Free | 356255 | Ceturegel™ basement membrane matrix for Organoid Culture |