Pseudo UTP Tris Solution GMP-grade (100 mM)
Description
Pseudouridine-5-triphosphate solution is one of the most commonly used modified nucleoside triphosphates. It is mostly used as the reaction substrate or coenzyme of enzymes, such as in vitro transcription, RNA amplification, siRNA synthesis, etc. The modified mRNA containing pseudouridine has better nuclease stability and translation characteristics, and reduces its immunogenicity. It has a wide range of applications in the field of therapy and diagnosis.
This product is produced in accordance with GMP process requirements and provided in liquid form.
Specification
|
CAS No. |
1175-34-4 (free acid) |
|
Molecular formula |
C9H15N2O15P3 (free acid) |
|
Molecular weight |
484.14 g/moL (free acid) |
|
Purity |
≥ 99% |
|
Content |
100 mM ± 3 mM |
|
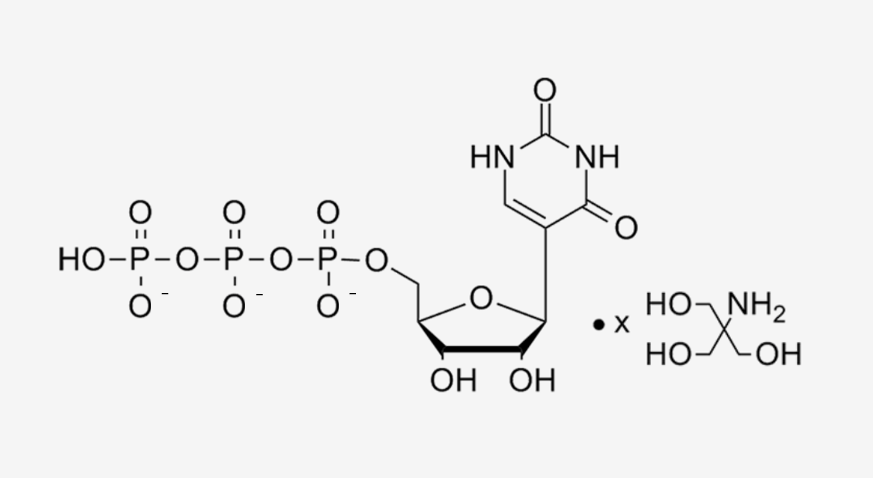
Structure |
|
Component
|
Components No. |
Name |
10656ES20 |
10656ES70 |
10656ES80 |
10656ES92 |
10656ES98 |
|
10656 |
Pseudo UTP Tris Solution GMP-grade (100 mM) |
20 μL |
100 μL |
1 mL |
10 mL |
100 mL |
Storage
The product should be stored at -25℃ ~ -15℃ for two years.
Notes
1. It can be dissolved at room temperature. After dissolution, it should be stored in an ice box or on an ice bath. After use, it should be stored at -20°C immediately.
2. For your safety and health, please wear personal protective equipment (PPE), such as laboratory coats and disposable gloves, when operating with this product.
Catalog No.:*
Name*
phone Number:*
Lot:*
Email*
Country:*
Company/Institute:*